Science equations can be complex; many times, it takes hours to understand an equation with multiple properties. It is an amazing subject, but it can terrify you if you don’t understand it properly. Many students pass their academics by merely memorizing the answers, avoiding paying attention, and making efforts to learn them. In this post, we will break down “HCOOCH CH₂ H₂O” and will discuss its structure, properties, and applications.
Chemical Structure And Properties
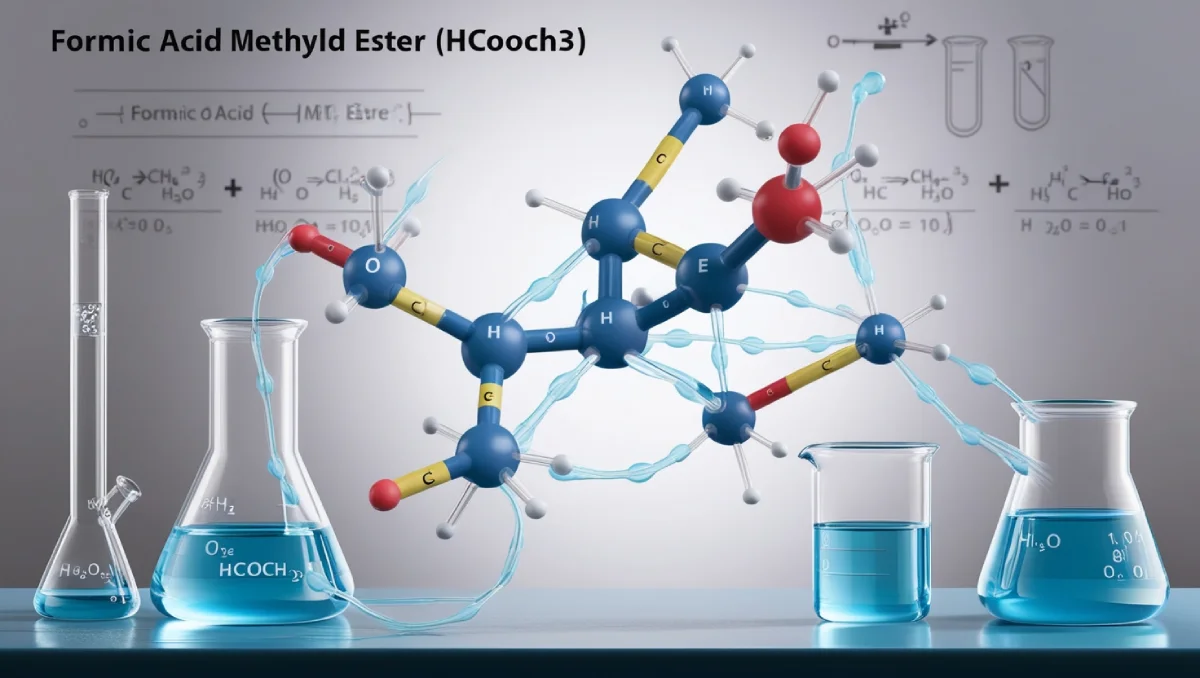
HCOOCH CH₂ H₂O is known as formic acid methyl ester in aqueous solution. It plays a vital role in multiple chemical reactions. Thanks to its unique structure, it is prone to react with both acid and ester. With a one-way reaction channel, chemicals can form numerous things; it is reactive with both acid and ester, which makes it a great solution for synthetic reactions.
Its properties are as follows:
- HCOO is the formate group derived from formic acid (HCOOH).
- CHCH₂ indicates the presence of an alkyl chain with a -CH₂ group attached to a -CH group.
- H₂O refers to water.
Applications Of HCOOCH CH₂ H₂O
HCOOCH CH2 H2O plays a crucial role in generating chemical reactions for various industries. It is widely used in the following industries:
- Pharmaceutical sector
- Agricultural industry
- Food processing
- Cosmetics production
Apart from these four industries, it is also used in other industries in the process of manufacturing and production.
Conclusion
Science is an interesting subject; it is an umbrella term, and various interesting topics come under it, such as chemistry. Learning about chemical reactions is both fun and tricky; it is important to pay attention to every element when it comes to understanding an equation. In this post, we discussed the HCOOCH CH2 H2O equation. We hope you found this helpful and will share it with other learners.